Well, as you can see, the brand of the lotion that I heart is Vaseline. Why I choose Vaseline? Because as I compared Vaseline with others brand, this lotion is easier to be absorbed into skin and leave a non-oily skin surface. Vaseline Company has a series of lotions with different functions. This ‘Healthy White UV Protection Lotion’ is one of my favorite lotions. This type of lotion contains yogurt serum, Vitamin B3 and triple sunscreen. I apply it every morning after shower. Yogurt serum in this lotion moisture and provide nutrition to my skin. Vitamin B3 helps in enhancing my skin’s natural lightning process leading to whiter skin that glows with health. In addition, triple sunscreen helps prevent skin dullness and dark spots caused by damaging UVA and UVB. UVB rays will cause our skin to become darker and generates the production of freckle while UVA rays accelerate the aging process and making a permanent white spot or brown spot. Although sunlight provides essential Vitamin D to our skin but the amount is considerable.
Different types of lotion gives different effects on skin, therefore, the ingredient to make lotion is also different. Ingredients used to make ‘Healthy White UV Protection Lotion’ are water, Isopropyl Myristate, Niacinamide 3.0% w/w, Stearic Acid, Glyceryl Stearate, Mineral Oil, Ethylhexyl p-Methoxycinnamate 1.25% w/w, Glycerine, Triethanolamine, Phenoxyethanol, Dimethicone, Butyl Methoxydibenzoylmethane 0.4% w/w, Carbomer, Cetyl Alcohol, Methylparaben, Sodium PCA, Glutamic Acid, Titanium Dioxide 0.2% w/w, Yogurt Powder 0.2% w/w, Fragrance, Propylparaben, Sodium Hydroxide, Disodium EDTA, Glycine Soja (Soyabean) Sterols and Lecithin. Total of 24 chemicals is needed to mix with water to make lotion. Therefore, I need to find out the function of these 24 chemicals in this lotion.
Different types of lotion gives different effects on skin, therefore, the ingredient to make lotion is also different. Ingredients used to make ‘Healthy White UV Protection Lotion’ are water, Isopropyl Myristate, Niacinamide 3.0% w/w, Stearic Acid, Glyceryl Stearate, Mineral Oil, Ethylhexyl p-Methoxycinnamate 1.25% w/w, Glycerine, Triethanolamine, Phenoxyethanol, Dimethicone, Butyl Methoxydibenzoylmethane 0.4% w/w, Carbomer, Cetyl Alcohol, Methylparaben, Sodium PCA, Glutamic Acid, Titanium Dioxide 0.2% w/w, Yogurt Powder 0.2% w/w, Fragrance, Propylparaben, Sodium Hydroxide, Disodium EDTA, Glycine Soja (Soyabean) Sterols and Lecithin. Total of 24 chemicals is needed to mix with water to make lotion. Therefore, I need to find out the function of these 24 chemicals in this lotion.
1) Water molecule is made up of H2Oand has a molecular weight of 18.02. It is a polar molecule and has a pH value of 7. The most common bond formed within water molecules are hydrogen bond. In lotion, water is fundamentally essential to enhance the restoring of flexibility of skin’s dry surface layers.
Structural formula of water molecules
2) Isopropyl Myristate is deriving from palm oil and is used as an emollient and lubricant in lotion. This chemical acts as a synthetic moisturizer and is absorbed readily by the skin. Isopropyl Myristate is a fatty acid which has a molecular formula of C17H34O2 and a formula weight of 270.45. The used of Isopropyl Myristate helps in promoting the production of acne. Therefore, if you have an oily skin type, I personally not suggest you to use this lotion.
Structural formula of Isopropyl Myristate
3) Niacinamide 3.0% w/w. Niacinamide is one of the Vitamin B3 compound. This chemical is used to improve the texture and tone of the skin, reduce fine lines, wrinkles and hyperpigmentation. Niacinamide has a molecular formula of C6H6N2O and a molecular weight of 122.247.
Structural formula of Niacinamide
4) Stearic Acid is a saturated fatty acid which also known as Octadecanoic Acid. The molecular formula for strearic acid is C18H36O2 and a molecular weight of 284.48. Stearic acid acts as an emulsifier which helps to hold the lotion together. This chemical keep all the other ingredients blended together in a smooth, creamy lotion.
Structural formula of Stearic Acid
5) Glyceryl Stearate is derived from palm kernel. This chemical has a molecular formula of C21H42O4 and a molecular weight of 358.56. Glyceryl Stearate acts as an emulsifier and functions same as Stearic Acid.
Structural formula of Glyceryl Stearate
6) Mineral Oil has a general molecular formula of CnH2n+2. Mineral oil appears as liquids at room temperature and if appears in solid form, is called paraffin wax. Mineral oil acts as an emollient which helps in moisture skin and gives skin a smooth feel.
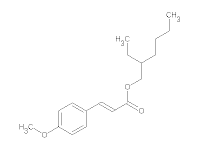
7) Ethylhexyl p-Methoxycinnamate (EHMC) has a molecular formula of C18H26O3 and a molecular weight of 290.403. EHMC is used as sunscreen agents.
Structural formula of EHMC
8) Glycerine is alternate name for glycerol and it is an organic compound. The molecular of Glycerine is
C3H5(OH)3. Glycerine acts as a humectants or “water magnet” that binds with water molecules and holds them in skin cells. Glycerine effectively helps in replicates the role of the skin own natural moisturizing factor.
Structural formula of Glycerine
9) Triethanolamine is an organic chemical compound. This chemical has a molecular formula of C6H15NO3 and a molecular weight of 149.188. Triethanolamine used as an emulsifier and surfactant. It also serves as a pH balancer.
Structural formula of Triethanolamine
10) Phenoxyethanol is an organic compound and is often used in dermatological products. It has a molecular formula of C8H10O2 and a molecular weight of 138.1638. It acts as a preservative that essential to keep lotion fresh every time user wants to use.
Structural formula of Phenoxyethanol
Structural formula of Dimethicone
Structural formula of Butyl Methoxydibenzoylmethane
14) Cetyl Alcohol is a fatty alcohol with a molecular formula of C16H34O and a molecular weight of 242.44. Cetyl Alcohol is used widely in dermatological industry as a surfactant, emollient and thickening agent.
Structural formula of Cetyl Alcohol
15) Methylparaben has a molecular formula of C8H8O3 and a molecular weight of 152.15. It acts as a preservative in lotion.
Structural formula of Methylparaben
16) Sodium PCA has a molecular formula of C5H6NNaO3 and a molecular weight of 151.096. It helps to hold water molecules in skin cells and keep skin hydrated.
Structural formula of Sodium PCA
17) Glutamic Acid has a molecular formula of C5H9NO4 and a molecular weight of 147.13. Glutamic Acid helps in maintaining skin elasticity and improving allergy.
Structural formula of Glutamic Acid
Structural formula of Titanium Dioxide
20) Fragrance is a chemical compound which also known as aroma. It gives out odour. Different type of compound gives different odour.
21) Propylparaben has a molecular formula of C10H12O3 and a molecular weight of 180.20. It acts as a preservative.
Structural formula of Propylparaben
Structural formula of Sodium Hydroxide
23) Disodium EDTA is a polyamino carboxylic acid. Is has a molecular formula of C10H14N2O8Na2 and a molecular weight of 336.22. It acts as a chelating agent and a viscosity adjuster. This can stable the ingredient from and form a stable product.
Structural formula of Disodium EDTA
25) Lecithin is composed of phosphoric acid, choline, fatty acids, glycerol, glycolipids, triglycerides, and phospholipids. It has a molecular formula of C46H89NO8P+ and a molecular weight of 815.175. It is an important component to build healthy cell membranes.
Structural formula of Lecithin
As a conclude, this lotion suits me because I always go for outdoor activities. With the UV protection in this lotion, I won’t get dark easier right? ^^
REFERENCES:
1) Gillespie Mary, 1999. UVA-UVB sun rays [online]
Available from :< http://www.911skin.com/uvbubarays.html >
[Accessed on 24.07.2010]
2) Hewson Susie, 2010. Isopropyl Myristate. [online]
Available from :< http://www.natracare.com/p119/en-GB/Your-Health/Chemicals-in-body-care/Isopropyl-myristate.aspx >
[Accessed on 24.07.2010]
3) Chemicalbook, 2007. Isopropyl Myristate. [online]
Available from :< http://www.chemicalbook.com/ChemicalProductProperty_EN_CB9482638.htm >
[Accessed on 24.07.2010]
4) Helmenstine Anne Marie, 2010. Vitamin B3 Niacinamide. [online]
Available from :< http://chemistry.about.com/od/imagesclipartstructures/ig/Vitamin-Chemical-Structures/Vitamin-B3--Niacinamide-.htm >
[Accessed on 24.07.2010]
5) About.com, 2010. Stearic Acid. [online]
Available from :< http://chemistry.about.com/od/factsstructures/ig/Chemical-Structures---S/Stearic-Acid-Octadecanoic-Acid.htm >
[Accessed on 24.07.2010]
6) Bogdan Allemann; L. Baumann, 2008. Antioxidants Used in Skin Care Formulations: Niacinamide. [online]
Available from :< http://www.medscape.com/viewarticle/582103_15 >
[Accessed on 24.07.2010]
7) Chemyq,2010. Glyceryl Stearate. [online]
http://www.chemyq.com/En/xz/xz11/106600kuelo.htm
[Accessed on 25.07.2010]
8) Wikimedia, 2010. Paraffin. [online]
Available from :< http://en.wikipedia.org/wiki/Paraffin >
[Accessed on 25.07.2010]
9) rdChemicals,2010 Ethylhexyl p-Methoxycinnamate. [online]
Available from :< http://www.rdchemicals.com/chemicals.php?mode=details&mol_id=3013>
[Accessed on 25.07.2010]
10) Helmenstine Anne Marie, 2010. Glycerine or Glycerin. [online]
Available from :< http://chemistry.about.com/od/factsstructures/ig/Chemical-Structures---G/Glycerine-or-Glycerin.htm>
[Accessed on 25.07.2010]
11) Wikimedia, 2010. Triethanolamine. [online]
Available from :< http://en.wikipedia.org/wiki/Triethanolamine >
[Accessed on 25.07.2010]
12) PubChem, 2010. Phenoxyethanol. [online]
Available from :< http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=31236 >
[Accessed on 25.07.2010]
13) PubChem, 2010. Dimethicone. [online]
Available from :< http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=24764 >
[Accessed on 25.07.2010]
14) Wikimedia, 2010. Cetyl Alcohol. [online]
Available from :< http://en.wikipedia.org/wiki/Cetyl_alcohol#Uses >
[Accessed on 25.07.2010]
15) chemBlink, 2010. Methylparaben. [online]
Available from :< http://www.chemblink.com/products/99-76-3.htm >
[Accessed on 25.07.2010]
16) PubChem, 2010. Nalidone . [online]
Available from :< http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=23668602 >
[Accessed on 25.07.2010]
17) Wikimedia, 2010. Glutamic Acid. [online]
Available from :< http://en.wikipedia.org/wiki/Glutamic_acid#Pharmacology >
[Accessed on 25.07.2010]
18) Wikimedia, 2010. Titanium Dioxide. [online]
Available from :< http://en.wikipedia.org/wiki/Titanium_Dioxide#Applications >
[Accessed on 25.07.2010]
19) chemBlink, 2010. Propylparaben. [online]
Available from :< http://www.chemblink.com/products/94-13-3.htm >
[Accessed on 25.07.2010]
20) Wikimedia, 2010. Sodium Hydroxide. [online]
Available from :< http://en.wikipedia.org/wiki/Sodium_Hydroxide#Domestic_uses >
[Accessed on 25.07.2010]
21) Cichanowski, 2002. The EDTA Story. [online]
Available from :< http://www.hbci.com/~wenonah/riddick/edta.htm >
[Accessed on 25.07.2010]
22) Wikimedia, 2010. Lecithin. [online]
Available from :< http://en.wikipedia.org/wiki/Lecithin >
[Accessed on 25.07.2010]
23) PubChem, 2010. Phospholutein . [online]
Available from :< http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6850739 >
[Accessed on 25.07.2010]
24) Biology, 2003. The Chemistry of Water. [online]
Available from :< http://www.biology.arizona.edu/biochemistry/tutorials/chemistry/page3.html >
[Accessed on 25.07.2010]
25) Vaseline Skin Fund, 2010. Ingredient Glossary. [online]
Available from :< http://www.vaseline.com/Template2.aspx?Path=Consumer/Glossary/Home >
[Accessed on 25.07.2010]



















No comments:
Post a Comment